
This week, we are excited to feature a guest blogger, Bryan Morgan. Bryan is a Strategy Consultant at Morgan Consulting, LLC, with 20 years of experience in the pharmaceutical industry, including the past 15 years working for biologic drug manufacturers. You can connect with Bryan here.
The world of medicine is rapidly evolving, bringing groundbreaking therapies to patients worldwide. At the heart of this transformation are biologic drugs—complex, life-saving treatments developed to tackle some of humanity’s most challenging health conditions. But behind these medical miracles lies a sophisticated and intricate manufacturing process, one that demands precision, scalability, and innovation. This is where HMI/SCADA (Human-Machine Interface/Supervisory Control and Data Acquisition) software with rule-based expert system steps in, assisting biologic drug production through advanced monitoring, automation, and control systems.
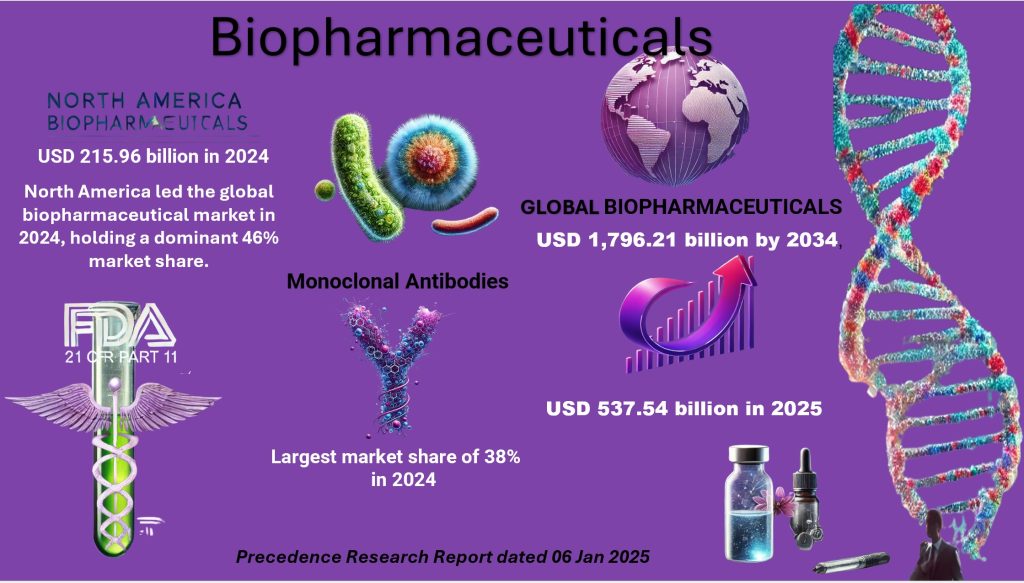
Data from https://www.precedenceresearch.com/biopharmaceutical-market
The global biopharmaceuticals market, valued at approximately USD 537.54 billion in 2025, is projected to soar to around USD 1,796.21 billion by 2034, with a robust compound annual growth rate (CAGR) of 14.36% from 2025 to 2034. This rapid expansion is driven by several factors, including the rising prevalence of chronic diseases, an aging population, advancements in biotechnology, increased demand for personalized medicine, government initiatives, and the ability of biologics to command premium pricing. Additionally, the growing focus on innovative therapies such as biosimilars and cell therapy is expected to significantly shape the market’s future.
While the biologics market holds immense potential, manufacturing these complex drugs presents unique challenges that distinguish them from traditional pharmaceutical production.
Key challenges include:
1. Complexity of Production
Biologics are large, complex molecules often produced using living cells or organisms, which makes their production significantly more intricate. Precise control of environmental factors such as temperature, pH, and nutrient levels is critical. The process involves multiple stages, including cell line development, fermentation, purification, and formulation—each with distinct challenges requiring advanced monitoring and automation.
2. Stringent Regulatory Standards
Given their complexity and potential risks, biologics are subject to rigorous regulatory oversight. Regulatory agencies, such as the FDA in the United States, mandate extensive testing and thorough documentation to ensure safety and efficacy. Compliance with these stringent standards adds to the complexity, time, and cost of developing and manufacturing biologics.
3. Quality Control and Consistency
Ensuring consistent quality and purity is paramount in biologics production. Even minor variations in the production process can significantly impact a drug’s safety and efficacy. Achieving batch-to-batch consistency requires sophisticated analytical techniques and rigorous quality control measures, often supported by advanced HMI/SCADA systems.
4. Scaling Up Production
Transitioning from small-scale laboratory production to commercial-scale manufacturing poses a significant challenge. Maintaining the consistency and quality of biologics at larger scales demands meticulous process control, robust data management, and specialized equipment, all of which increase production complexity.
5. High Production Costs
The intricate production processes, stringent regulatory requirements, and need for specialized equipment make biologics significantly more expensive to produce compared to traditional pharmaceuticals. These high costs can limit patient access, particularly in developing regions where healthcare resources are scarce.
Overcoming Challenges with Advanced Technology
Despite these challenges, the adoption of advanced technologies such as HMI/SCADA systems with integrated rule-based expert systems can streamline manufacturing, improve consistency, and reduce costs. By addressing critical areas such as real-time process monitoring, regulatory compliance, and quality control, these technologies empower manufacturers to meet the growing demand for biologics while maintaining the highest safety and efficacy standards.
Complexity of Production
Biologic drugs are developed to treat complex health issues like cancer, diabetes, and immune disorders. How biologic drugs are produced differs greatly from how pharmaceutical drugs are produced. Pharmaceutical drugs are small molecules consisting of dozens of atoms each, and manufacturers synthesize them using fundamental chemical reactions. Biologic drugs are large molecules consisting of thousands of atoms each and are produced from modified living cells, called a cell line. A cell line generates compounds as part of its life cycle. Once a cell line has been created to produce an effective biologic drug, manufacturers can replicate billions of identical copies to consistently produce effective biologic drugs at scale.
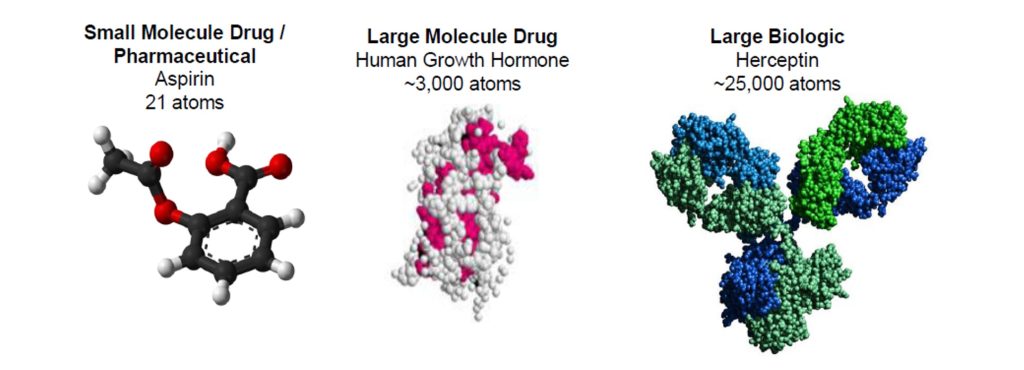
Image from: https://www.edisongroup.com/thematic/the-a-b-t-of-biosimilars-explaining-contemporary-life-science-issues/
A Delicate Process:

Genentech lab technicians in the second cell culture manufacturing facility clean room check the status of cell growth in eight large stainless steel 25,000-liter bioreactors (at left) that will ultimately become antibody medications to treat certain types of blood cancers.
Manufacturing biologic drugs is a complex process that must be constantly monitored, effectively maintained, and actively controlled. The entire process occurs in a bioreactor, similar to wine fermentation vats. Within each bioreactor billions of modified cells live out their life cycles breathing, feeding, expelling waste, replicating, and dying while producing the biologic drug that will be harvested by the manufacturer. It is a delicate process with hundreds of factors (including temperature, pressure, pH, oxygen, carbon dioxide, nutrients, and waste levels that must be constantly monitored, adjusted, and recorded. Even a slight variance in one of these factors can have a profound impact on the drug yields or, worse, make the drug ineffective.
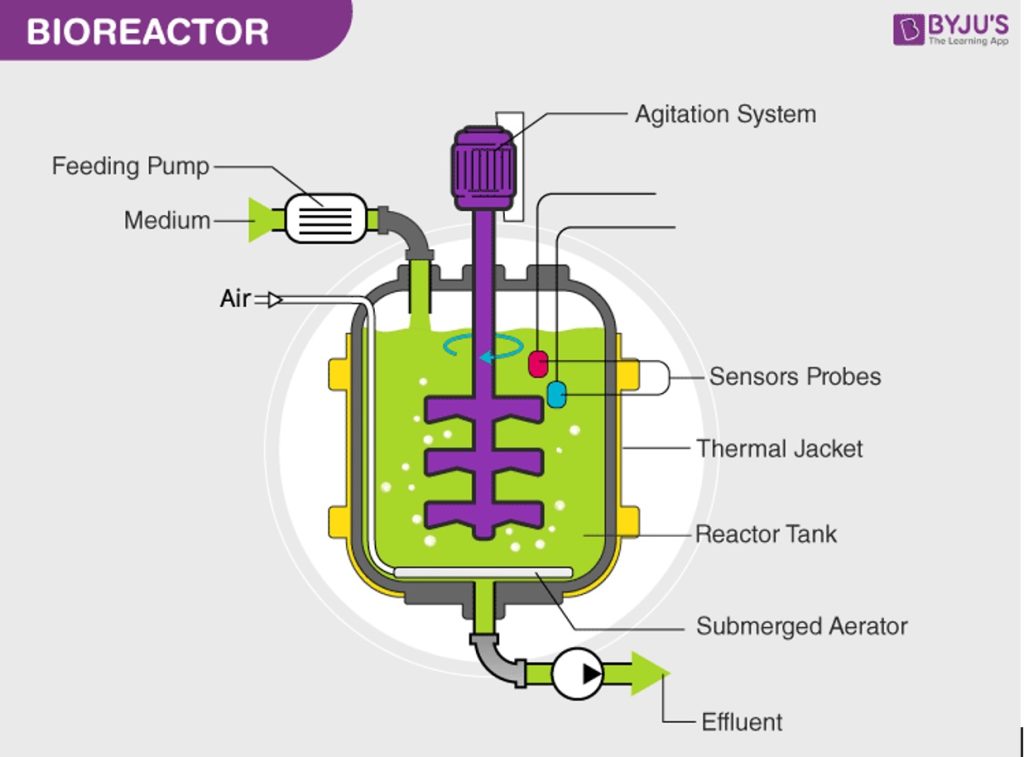
Image from https://byjus.com/biology/bioreactor-obtaining-foreign-gene/
Even the Wind Matters: Unveiling Hidden Factors in Biologic Drug Manufacturing
Several years ago, a biologic drug production facility faced a perplexing challenge: one of its production lines was delivering inconsistent yields. Some batches yielded effective drugs as expected, while others failed to meet the necessary efficacy standards. The mystery deepened when they found that all input variables tracked by the HMI/SCADA system were identical, operational ranges were within specifications, and yet, the results were wildly different.
After extensive investigation, they uncovered a surprising culprit—the direction of the wind outside the building. Successful batches were consistently produced when the wind blew from the east, while failed batches occurred when the wind came from the west. It turned out that the facility’s exhaust vent was positioned west of the air intake vent on the roof. When the wind blew eastward, exhaust contaminants were carried into the air intake vent, compromising the production process.
This discovery led to a critical enhancement in their operations. Today, the air intake quality is a vital data point continuously monitored by the HMI/SCADA system, ensuring a controlled environment and consistent production outcomes.
HMI/SCADA in Biological Drug Manufacturing
In the complex world of biologic drug production, ensuring safety, efficacy, and consistent quality is paramount. HMI/SCADA software is vital in achieving these goals by providing advanced monitoring, control, and automation capabilities. These systems are indispensable for maintaining the precision required to produce life-saving treatments that address a wide range of diseases. From tracking critical environmental conditions to managing intricate production workflows, HMI/SCADA ensures that every step of the manufacturing process meets the highest standards of reliability and compliance.
This precision and control not only ensure the quality of biologic drugs but also lay the foundation for scalable and adaptable manufacturing processes. To meet the growing demand for these life-saving treatments, HMI/SCADA systems must go beyond monitoring—they must enable reliable, coordinated, and compliant operations at every scale of production. Some essential features of an HMI/SCADA system for biologic drug manufacturing include:
- Scalable: Recipes specify time, temperatures, pH, ingredients, process, and guardrails to standardize production at multiple facilities.
- Adaptable: Standardized templates can be quickly translated to utilize another language and/or system of measurement.
- Reliability: Advanced alarms and a rule-based expert system enable automated responses, allowing the system to detect variances and adjust parameters in real-time, minimizing waste and reducing the need for rework.
- Coordination: Seamless communication with upstream and downstream processes maximizes efficiency, minimizes downtime, and ensures an uninterrupted flow of production. Additionally, integrating a rule-based expert system for predictive maintenance further reduces downtime by addressing potential issues before they impact operations.
- Compliant: Capturing data from sensors, alarms, and manual inputs in real-time results in seamless documentation for dashboards, reporting, process improvement, audits, and regulators.

Every Detail Matters: Data Capture and FDA 21 CFR Part 11 Compliance in Biologic Drug Manufacturing
Every step in the biologic drug manufacturing process directly impacts the drug’s quality and, ultimately, patient outcomes. Data capture is a critical component across all stages—from production and packaging to storage, distribution, and even administration. Each management action and process must be meticulously documented to meet stringent industry standards and ensure regulatory compliance.
Adhering to FDA 21 CFR Part 11, manufacturers must implement robust systems that ensure the integrity, authenticity, and accuracy of electronic records. Real-time data capture and utilization are essential for identifying and addressing potential issues during production that could affect product quality and patient safety. This capability is vital not only for delivering consistent, high-quality products but also for meeting the expectations of healthcare providers, public health officials, regulators, governments, executives, investors, and auditors.
Regulators often conduct unannounced audits, requiring manufacturers to maintain thorough, up-to-date documentation that demonstrates all issues have been identified, addressed, and resolved. Compliance involves more than just regulatory assurance; it extends to leveraging data to uncover the most efficient production parameters, enabling manufacturers to deploy materials, machines, and personnel effectively. This includes reducing waste, optimizing preventive maintenance schedules, and ensuring proper oversight and management throughout the production lifecycle.
Manufacturers also use this data to identify efficiencies and improve processes, creating a competitive advantage and delivering value to investors. By aligning with FDA 21 CFR Part 11 standards and integrating advanced data capture and analytics systems, manufacturers can ensure compliance, maintain quality, and drive innovation in biologic drug production.
If you are interested in how regulators like the FDA want manufacturers to document their manufacturing process in real-time, read ADISRA’s previous blog on FDA 21 CFR Part 11 here.
Optimizing Biotech Manufacturing with HMI/SCADA and Rule-Based Expert Systems
HMI/SCADA applications with rule-based expert systems are revolutionizing biotech manufacturing by optimizing key areas such as formulation design, process control, quality assurance, and predictive maintenance. These processes often involve millions of discrete data points and rely on biologic elements—living cells or their components—making precision and control essential.
While biotech companies tackle digital biotechnology from various perspectives, this blog will explore two critical use cases:
1. Process Monitoring and Control
Real-time data analysis powered by HMI/SCADA expert systems can detect deviations from desired manufacturing parameters, enabling swift corrective actions to maintain product quality.
For instance, imagine a manufacturing process that requires strict temperature control. Operators monitor real-time temperature data using HMI/SCADA systems, such as ADISRA SmartView, which incorporates expert system capabilities. These systems continuously analyze data and flag deviations, instantly alerting operators to potential issues.
If the temperature drifts outside the acceptable range, operators can quickly adjust settings to restore optimal conditions. This automated approach ensures consistent product quality, effectively acting as a digital quality control mechanism that safeguards each product batch.
2. Quality Assurance and Regulatory Compliance
HMI/SCADA expert systems are invaluable in analyzing quality control data and identifying potential compliance risks, ensuring products meet stringent regulatory standards.
ADISRA SmartView incorporates Statistical Process Control (SPC) within its trending module to strengthen quality control efforts. SPC enables manufacturers to identify and eliminate process variations by tracking key parameters and leveraging statistical analysis. This approach allows for pinpointing the root causes of variation, enabling corrective actions that enhance product quality, reduce defects, and boost customer satisfaction. Additionally, the integration of a rule-based system ensures compliance by monitoring standards and triggering alarms or alerts when the system deviates from regulatory requirements. Together, these tools provide a robust framework for maintaining the highest quality in biomedicine manufacturing.
This built-in SPC capability empowers manufacturers to meet quality and compliance requirements seamlessly, reinforcing confidence in their processes and products.
Experience ADISRA SmartView firsthand by downloading a free trial from our website here. See how our HMI/SCADA solution can transform your manufacturing processes, delivering precision, efficiency, and reliability in biotech and beyond.
Conclusion: Driving Innovation in Biologic Drug Manufacturing with HMI/SCADA and Rule-Based Expert Systems
The integration of HMI/SCADA applications and rule-based expert systems has become a game-changer in biotech manufacturing, addressing the industry’s need for precision, efficiency, and regulatory compliance. By enabling real-time process monitoring, automated quality control, and proactive maintenance, these advanced systems optimize critical manufacturing processes and ensure consistent product quality.
As demonstrated, tools like ADISRA SmartView empower manufacturers to streamline operations, enhance quality assurance, and adhere to stringent compliance standards, such as FDA 21 CFR Part 11. These capabilities not only safeguard the integrity of biologic drug production but also provide a competitive edge in a highly regulated and demanding industry.
Biologic drug manufacturers striving for innovation and excellence can leverage the power of HMI/SCADA systems to transform their processes and meet the growing demands of the healthcare market. Experience the impact firsthand—download a free trial of ADISRA SmartView from our website today and take the first step toward revolutionizing your manufacturing operations.
ADISRA®, ADISRA’S logo, InsightView®, and KnowledgeView® are registered trademarks of ADISRA, LLC.
© 2025 ADISRA, LLC. All Rights Reserved.
